For all fresh fish, liver and meat preparations, the right knowledge of the pH-value is the most important aspect to assign the proper objective to the fresh or prepared product. The importance of the pH-value for all fresh fish and muscle meat is based on the fact that the properties of fish and muscle meat are clearly influenced. Thanks to our 35 years of practical experience, an improved buffer pH-value is used for all fresh fish and muscle meat preparations. This is done with pH Liquid Extract® 601/6014/60148 in conjunction with the range of Buffer Phosphates 60150-60151/60152.
See below for explanation with regard to ’PH Liquid Systems and Buffer films’ with an improvement of colour, taste, smell, storage life, tenderness and water-binding capacity. By applying a buffer pH-value, we also guarantee a double storage life for fresh prepackaged products, both heated and non-heated. As a result, no generation of gases occurs in the packagings. The universally known pH-values and their importance for all fresh fish, liver and muscle meat preparations.
In order to define the pH-value correctly, it must be examined in greater depth
In plain words, the pH-value is usually defined as ’degree of acidity’. However, this is not correct for two reasons. Firstly, non-acid solutions also have a pH-value. Secondly, various organic acids also show different pH-values in case of equal acid concentration in solutions. We will discuss these reasons in detail below.
In order to explain what the pH-value is, we best start from pure water. To a very small extent, water (H2O) disintegrates into hydrogen ions, H+ and OH- ions. We define this spontaneous splitting as dissociation:

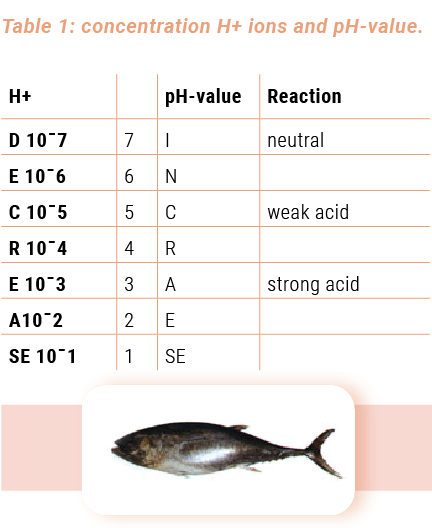
The number of H+ ions in pure water is very small and amounts to 0.0000001g per litre. This number can more easily be expressed as 10-7. Now; the pH-value is nothing more than the exponent (-7) without the minus sign. So the pH-value of water is 7. Since pure water is neutral, pH7 indicates the neutral point on the pH-value scale.
The mathematical aid to go from the number 10-7 to the number 7 is the so-called logarithm (abbreviated as log). We write log 10-7 = -7. If we multiply –7 by –1, we get +7 = -log 10-7 = 7.
If we replace the special concentration (10-7) by the general concentration of hydrogen ions (H+), we get the symbol –log (H+), that is defined as pH-value.
pH = -log (H+)
In words: the pH-value is the negative logarithm (i.e. the positive exponent) of the concentration of hydrogen ions.
Acids are substances that give off the H+ ions in water.

The higher the concentration of hydrogen ions, the lower the pH-value (see table 1).
Strong acids are acids that fully dissociate (e.g. hydrochloric acid). The oxygen concentration of strong acids is equal to the concentration of H+ ions. Weak acids (including most of the organic acids, such as acetic acid and lactic acid) dissociate only partially. That is why – in addition to the concentration – the dissociation constant K or rather pK (= -log K) is important as the standard for the degree of dissociation of weak acids. This is the reason for the above-mentioned fact, that the pH-value of meat (caused by lactic acid) is not equal to the degree of acidity or the acidity content. The pK-value of the various acids is different, so that many acid solutions also show different pH-values in case of an equal concentration.
The counterparts of the acids are the bases. Their pH-value is higher than 7. The more a solution is basic, the higher its pH-value is.
Connection between the pH-value and the acid/base reaction: see illustration 1.
ACID BASIC
________________________________________
Illustration 1: Connection between pH-value and acid/base reaction
The pH-value practically ranges between 0 and 14. For meat, only a small range of +/- pH 4.5 to pH7 is important. That is why measurements must be carried out very accurately in order to determine the pH-alterations and the pH-differences.
Next to acids and bases, the so-called buffers are also important for the pH-value. Buffers have the quality – to a certain extent – that they maintain a constant pH-value if they catch H+ ions (e.g. in case of addition or generation of acid). Such buffers contain the salts of weak acids, in meat e.g. of lactic acid (lactate). The negative salt anions combine the H+ ions through the combination of non-dissociative acid:

The proteins of the meat also act as buffers.
This means that the lactic acid, which is formed in the meat after the slaughtering, brings about a small decrease in the pH-value, as it would be the case in a pure watery solution.
The pH-value of a buffer depends on 3 factors: the acid concentration, the pH-value and the salt concentration:
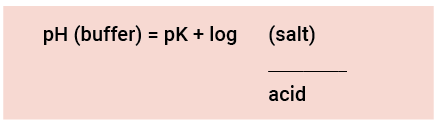
This comparison shows that the pH-value of buffers remains unchanged in case of a mild dilution with water, as the proportion salt/acid does not change. (The value of K
(= pK) is also constant in case of a mild dilution).
pH-value of muscle meat quality
The pH-value of a living muscle is situated slightly above the neutral point (pH 7.2). After the slaughtering (post mortem, p.m.), a process of biochemical degradation sets in. The carrier of energy of the muscle, the glycogen, is degraded into lactic acid through the action of different enzymes:

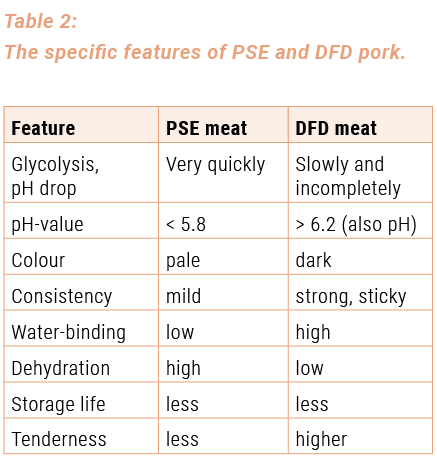
This process is called glycolysis. The pH-value of the meat decreases due to the forming of acid. In a normal situation, glycolysis happens slowly and the pH-value for pigs falls within 24 hours to a final pH-value of 5.8 and less (up to 5.3). On the other hand, if the glycolysis happens very quickly and the pH-value drops below 5.8 within a period of 45 minutes (the so-called pH1-value), this is characteristic of PSE meat. PSE meat has a low water-binding capacity. However, it is also possible that there is only a slight decrease of the pH-value of the meat (after the slaughtering) owing to a lack of glycogen. If after 24 hours the pH-value is still higher than 6.2, we are dealing with DFD meat. DFD meat has a high water-binding capacity, a dark flesh-colour and a short storage life (see table 3). The rate at which the pH-value falls and the occurrence of PSE and DFD qualities of the meat can be due to genetic reasons or to stress experienced by the animals before and during the slaughtering. The pH-evolution for normal, PSE meat and DFD meat can be found in illustration 2.
The characteristic features of PSE and DFD meat, that are decisive for the processing possibilities, can be found in table 2.
When the final pH-value has been reached, i.e. at the end of the glycolysis, the value remains unchanged for some time, and then increases slightly (+/- 0.1 pH-units) during the storage (during the ripening process). In case of long storage, bacteriological taint occurs and the pH-value increases considerably up to values of 6.5 due to the development of substances showing an alkaline reaction (e.g. ammonia). The complete pH-evolution from the slaughtering to the exceeding of the storage life, can be found in illustration 3.

The specific pH-evolution during the storage of a particular kind of meat is influenced by many factors, especially by the temperature, the bacteriological status (germ number and type) of the surrounding atmosphere (air, CO2, vacuum packaging) and the type of meat. Cooling slows down and freezing stops the post mortem alterations in the meat.
Processing possibilities of PSE and DFD meat
Although the qualities of PSE and DFD meat differ substantially from those of normal meat, they are fit for the various preparation and processing purposes. The technological qualities of PSE and DFD meat are formulated in table 2 in view of their suitability for the production of meat products. The most important pH-values of fresh meat, that are particularly important for the choice of the raw material, can be found in table 3.
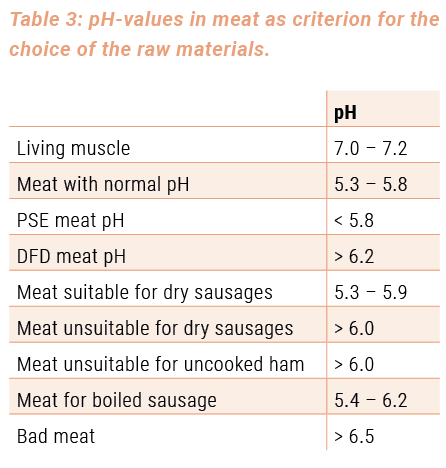
A choice of meat products, which can be prepared with PSE and DFD meat, that balances this fault, is listed in table 4.
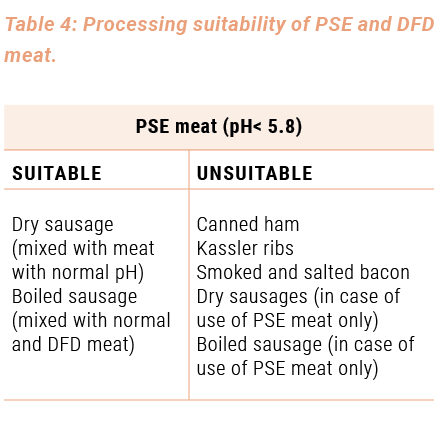
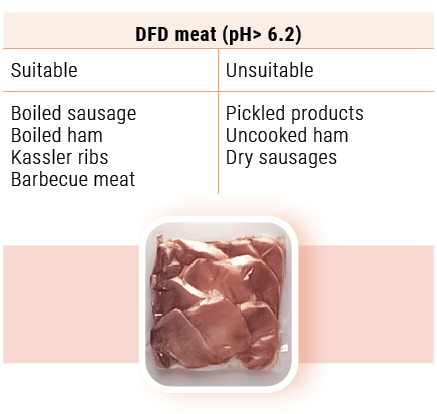
The reason for the suitability or the unsuitability of PSE and DFD meat for the various processing purposes is clear from the qualities mentioned in table 2. For instance, PSE meat is not suitable for the production of boiled sausage, boiled ham (increased jelly deposition) and uncooked ham (high loss of weight, pale colour and little aroma) because of its low water-binding capacity, whereas DFD meat has an excellent water-binding capacity, but is not recommended because of its perishableness (high pH-value for uncooked ham (especially ham on the bone)). The use of DFD meat only also involves a short storage life for dry sausage and boiled sausage. Processing this type of meat together with meat with a normal pH-value is advisable.
As you can see, it is possible to determine the proper choice of raw materials for the preparation of high-quality and non-perishable products by means of an inspection of the pH-value in the meat.
The pH-value of muscle meat products
The quality of the meat largely depends on the quality of the prepared products, taking into account that the pH-value plays a very important part. For some quality factors, low pH-values are favourable (suitability for salting, storage life, taste). On the other hand, high pH-values are favourable for other factors (flesh-colour, water-binding capacity) (see table 5). For normal meat, these factors are balanced.
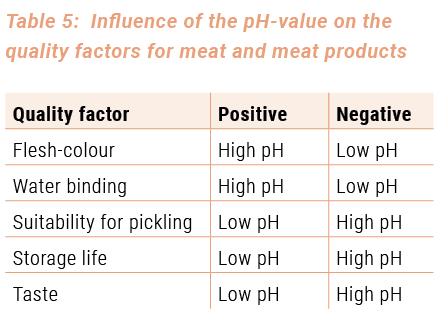
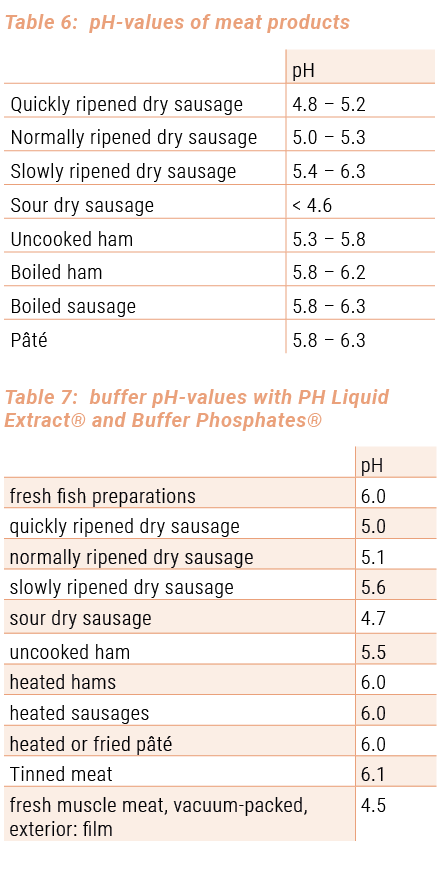
During the processing, the pH-value of the raw material ‚muscle meat’ changes due to the use of additives, the activity of micro-organisms (e.g. ripening of dry sausages) and the heat treatment of the meat, which usually leads to a higher pH-value. End products normally have other pH-values than the used raw materials. PH-values can also be given for meat products. Experience shows that they can be considered as normal (see table 6). These values can serve as reference values for quality control and assessment.
As a result, the pH-measurements can provide valuable information for fresh meat and most meat products with regard to the quality of the product concerned. Conversely, we can say that the product meets certain quality requirements if it has a normal pH (e.g. good hygiene – long storage life).
Summary
The influence of the buffer pH-value on the quality of fresh fish, liver and muscle meat and on all meat preparations is extremely important for the colour, the taste, the smell, the firmness, the water binding and the storage life.
The rate at and the intensity with which the pH-value falls after the slaughtering of the animal (caused by the formation of lactic acid) also strongly influence the processing qualities of the meat. If the pH-value falls too quickly, the meat will be watery, pale owing to poor binding, it will lack aroma and have a diminished storage life (PSE quality).

The pH-value of normal meat falls naturally, normally and fully and stabilises between
pH 5.4 – 5.8 24 hours after the slaughtering.
If you apply a correct buffer system, both to preserve fresh carcasses for slaughtering and muscle meat stripped of bones and membranes, that is ready to be vacuum-packed and has been treated before the packaging with a film of PH Liquid Extract and Buffer Phosphate 60152 until the exterior had a
pH-value of 4.5, you will obtain a very hygienic and stable, prepackaged buffer meat product.
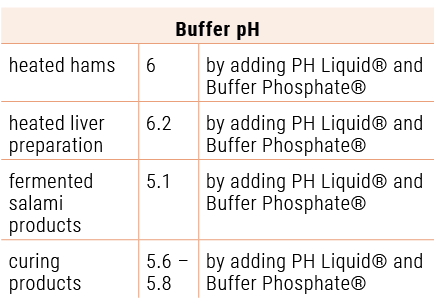
This also applies to all further production lines, such as heated hams, heated liver preparations, fermented salami products and all curing products. They obviously have an adjusted buffer pH-value:
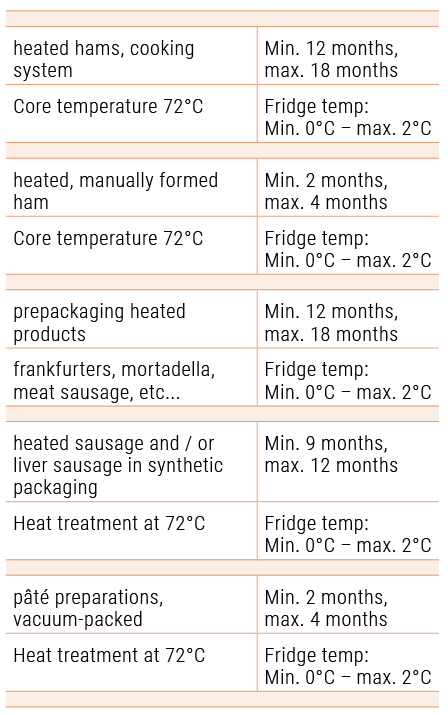
If you apply PH Liquid Buffer Systems and Buffer Phosphates at the same time and observe a storage temperature of 2°C, PH Liquid Belgium nv guarantees the following storage life:

Author: Walther Van Kerrebroeck / Meat Technologist for PH Liquid Belgium Nv

